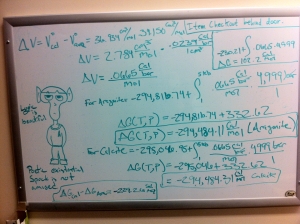One of the more heavily debated topics within the field of meteoritics is the origin of chondrules. These are the small, spherical silicate inclusions from where we derive the technical name for the most common type of meteorites, the chondrites. With few exceptions, chondrules are found in all chondrite groups in varying quantities. Sometimes we’ll see chondrites that are nearly 70% chondrules and in other cases, we’ll see chondrites, such the Ivuna, that contain no chondrules. In the simplest of terms, chondrules are composed of olivine and/or pyroxene, occasionally glass and a smattering of feldspar. In not so simple terms, chondrules are a hot mess of textures and compositions- messy enough that I’m not going to cover it in this post, but I did delve into it a bit in this older Meteorite Monday post about our enigmatic friends. Continue reading
Meteorite Monday: The Antarctic Search for Meteorites
Today’s Meteorite Monday will be a short one. I’m in the thick of finishing my senior project about the meteorite I’ve been working on for over a year and a half. Today I wanted to touch briefly on the NSF funded Antarctic Search for Meteorites (ANSMET). This is one of the most productive, publicly funded ventures in the hunt for meteorites. In its 37 years of existence, the scientists at ANSMET have recovered nearly 20,000 meteorites from the cold, barren ice-fields of the Antarctic. This continent is perfect for the recovery of meteorites because it’s dry, devoid of vegetation and sees little in the way of sediment build-up. All this leads to conditions where meteorites, black from their fusion crust, can easily be recognized against the white backdrop of the slow-moving ice.

One of the ~400 meteorites recovered from Antarctica during the 2012-2013 season. (Image from Planetary Science Research Division at the University of Hawai’i- Manoa)
Some famous meteorite alumni from past ANSMET expeditions include the first two lunar meteorites Yamato 791197 and Alan Hills 81005. The martian meteorite, Alan Hills 84001, was also recovered from Antarctica. This is the martian meteorite that was thought to have fossilized martian microbes. The first two digits of the number is the year that the meteorite was found, and generally speaking, the name, i.e., Alan Hills, is the region where it was found.
Once these meteorites are found, they’re sent to Johnson Space Center where they’re classified and made available for study by qualified meteoriticists and planetary science researchers.
Here’s a cool YouTube video showing how the work is done:
Meteorite Monday… er, Tuesday!
Believe it or not, I’ve missed my blog. I’ve missed interacting with my readers and talking about all the cool science occurring in meteoritics and planetary science. A few factors have contributed to my absence from blogging: school/work load, my work in the meteorite lab, and a general burn-out over the course of the academic year. I essentially worked myself into the ground during the Fall and Winter term and the Spring term has been my recovery period. That and it’s difficult to get back into blogging when you’ve been away from it for a period of time. I’m hoping to change that though with more regular posts.
So, as a way of easing back into the blog I thought I’d start with a new Meteorite Monday Tuesday! This time I’m going to talk a bit about a project that has consumed a great deal of my time: the shock dike in my meteorite. And since this is all available in the poster I did for LPSC, I don’t have to worry about revealing information intended for the publication.
Here’s a simple slide of the shock dike that I’ve been working on for the past year or so:
What you’re looking at is a transmitted light image of the meteorite I’ve been working on. That thick, black structure from the lower right corner to the top left corner is what we’re referring to as a shock dike. This isn’t something that’s been studied before, so our term isn’t one that’s been utilized in the past. In fact, I ran into some resistance to the term at LPSC because the term dike is traditionally used in a terrestrial setting and not that of an extraterrestrial. What some people forget is that we apply earth based geophysical and geochemical analogs to other bodies in the solar system all the time. It’s that application that allows us to understand the forces that shape the other rocky planets.
Here’s what we know about the formation of shock veins, and by extension, this shock dike. We know they form as a result of a collision in space. The kinetic energy from that collision would become the heat needed to melt a large portion of the rock. That melted rock acts a lot like water- it flows through cracks and exploits areas of weakness within the solid rock structure. That sudden influx of melted rock also drives up the pressure and changes the minerals it encounters into high pressure polymorphs- or minerals with the same chemical composition, but a more compressed crystal lattice structure. Such minerals become encased in the rapidly cooled vein and don’t have the chance to change back to their preferred structure.
What makes the shock dike unique is that it didn’t cool quickly and there are no high-pressure polymorphs. Everything pretty much melted. The heat needed to melt the rock was produced by an impact and sustained through friction as the rock sheared like a strike-slip fault. This sustained heat and mechanical grinding vaporized the less heat tolerant feldspars, and melted pieces of the more heat resistant pyroxenes and olivines. As these minerals broke down they added their chemical components to the melt. This, combined with a relatively long cooling time, caused new minerals to form out of this melt. Such minerals include pyroxenes composed of alternating bands or iron and magnesium and aluminum rich pyroxenes; all indicative of crystals that grew from a melt.
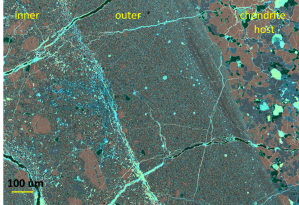
This is a false colored back scatter electron image of the shock dike. The salmon colored minerals in the inner dike are olivines and they correspond to the same minerals found in the chondrite host. The false color allows us to determine the chemistry of the minerals we’re looking at.
Now that we’ve got a handle on a the chemistry of our system and how it possibly formed, our next task is to deduce how long it took to cool. We know that it sustained its heat for a time sufficient enough to generate brand new crystals. What we don’t know is how long it took to cool. Something we’re looking at is the high aluminum content in our pyroxenes. The amount we’re dealing with tells us that we had some disequilibrium conditions occurring right as our melted rock solidified. This will be important for telling us the maximum temperature of our melt and the temperature at which it cooled.
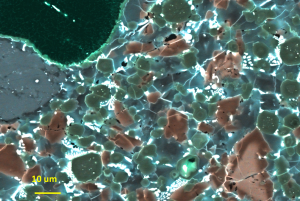
This is the same false color BSE image, but at a higher magnification. The bright green minerals are our aluminous pyroxenes. The large greyish blue clast in the upper left is a pyroxene clast and the one mineral right above it is a feldspar. The large triangular mineral in the lower left corner is an olivine clast. The bright pink core is unmelted iron rich olivine and the dark pink rims are melted and recrystallized magnesium rich rims.
This summer I’ll be working to address these issues through the McNair program. This is a research scholarship that will essentially pay me to do full time research this summer and present it at the McNair Symposium. If I’m so inclined (and I am) I can also submit it to the McNair Undergraduate Journal for publication. Then in the Fall I’ll be working with both my advisers on a journal publication about my meteorite. I’m also looking to do a poster for the AGU conference in December. If I’m feeling particularly courageous, maybe an oral presentation, too.
Further goodness I’ve been involved in:
- A Pyroxene Enriched Shock Dike in the Buck Mountains 005 L6 Chondrite (Hutson, M., Ruzicka, A., Brown, R. LPSC 2013. Abstract 1186)
- Stones from Mohave County, Arizona: Multiple Falls from the Franconia Strewn Field (Hutson, M., Ruzicka, A., Timothy Jull, A. J., Smaller, J. E. and Brown, R. (2013), Meteoritics & Planetary Science, 48: 365–389. doi: 10.1111/maps.12062
A glimpse into my home away from home, the Cascadia Meteorite Lab.
A couple months ago a student in PSU’s film department, Emily Yurek, decided to do a short documentary on the meteorite lab. In the documentary she interviews Dick Pugh, our awesome out-reach coordinator, where he talks about the lab and why we study meteorites. Don’t tell him I said this, but I have a lot of respect for Dick. He knows a lot about meteorites and is enthusiastic in sharing his knowledge. I was also interviewed and got to talk a bit about the work that I’ve been involved in.
I think the documentary turned out well and I’m grateful to Emily for making the lab a little more visible to the public. My hope is that this video will generate more publicity for our lab, and hopefully, donations. We have over 700 samples to study and not enough monetary resources to do the work. All the work is voluntary and done out of a love for the science of meteoritics. And since we are a publicly funded lab, we’re here to answer the public’s questions about meteorites and even look at rocks if you suspect you might have a meteorite.
Anyways, watch the documentary and, if you feel like chipping in even $5, click here. Every donation goes towards a lab that makes possible original research for undergraduates (such as myself) and graduates alike.
A few of my favorite moments from LPSC 2013
Last week I attended my first science conference: The Lunar and Planetary Science Conference in Houston, TX. If you followed me on Twitter, then (for better or for worse) you also knew that I was one of about 40 people microblogging the conference. There was a lot of great science to cover and it was fun trying to distill that information into 140 character limit. I got to present a poster on the shock dike that I’ve been working on for over a year now and I received good feedback on the research. I also met a lot of great people, some of whom will be potential collaborators in research and outreach projects.
At the end of the conference I began to think about my favorite aspects and topics at LPSC. There’s no rhyme or reason to this list, but a few of these items will become blog posts once I sort through all my tweets and notes and can write something coherent.
Conferences are not restful– I wasn’t expecting a vacation time, but nor was I expecting to consume the same amount of coffee as I would during finals. Between microblogging and networking, there wasn’t much downtime. At the end of the conference, I was pretty beat.
Scientists are real people– This may seem like a silly thing to say, but the outside world thinks we’re either socially inept like those in the Big Bang Theory or we’re protected by the walls of the Ivory Tower. True, some scientists don’t have the best social skills, but most that I met are great to hang out with, play games, and enjoy a beer or two. And the fragility of the Ivory Tower has never been more apparent until Sequestration hit. The funding cuts meant that many scientist couldn’t attend LPSC and present their research, collaborate with their peers, and participate in the free flow of information. More importantly, some are concerned about job security.
One of many favorite tweets from the conference:
https://twitter.com/DoctorApatite/status/314392557197094912
Pre-solar CAI’s– I nearly choked when I heard this in a presolar grains session. A CAI is a calcium aluminium rich inclusion. These things are composed of refractory elements, or elements that stay solid at very high temperatures. They are the oldest things in the solar system and condensed out of the solar nebula before the planet making process took off. Presolar indicates something that formed before our solar system. So, finding a probable presolar CAI is like sampling the proto nebula of another solar system. Pretty neat!
The MESSENGER mission at Mercury is churning out some of the best science in NASA- I’m of the opinion that the MESSENGER mission is kind of the underdog of the NASA missions. Cassini and anything Mars related get a lot of much deserved attention, but the people working on MESSENGER deserve accolades, as well. The lack of attention isn’t their fault- they have a fantastic website, but it’s hard to compete with the rings of Saturn and a laser shooting robot searching for past clues of life.
Mercurian meteorites– Few topics are as contentious as this one. A researcher from University of Washington claimed to *possibly* have a meteorite from the innermost planet, or at the very least, from a very similar planetesimal. Could we have a few Mercurian meteorites in our collection? Possibly. But current spectroscopic data from Mercury don’t align with any known meteorites. Another problem: crystallization age was revealed to be ~4.3 billion years old. This is too old to come from Mercury, but could possibly come from another asteroidal source.
The HED/4 Vesta debate– Another contentious topic in the meteoritics field. The HED’s are a group of meteorites that likely came from asteroid 4 Vesta. Spectral reflectance from the asteroid matches that of this suite of meteorites. However, a few within the meteoritics world will tell you that the science is wrong and one group of these meteorites, diogenites, may have come from two parent bodies. This would eliminate 4 Vesta from being the HED parent body. This is a fairly heretical stance because the link between HED’s and 4 Vesta is fairly well established, but still provides for some solid conference entertainment.
Platinum anomaly that may reignite the Younger Dryas impact hypothesis– The idea that the Younger Dryas extinction was caused by an asteroid impact has been a dead issue for the meteoritics community for some time now. The evidence wasn’t really there to support the hypothesis until a researcher found platinum anomalies in ice cores from Greenland. Now, he didn’t claim that it came from an impact, but the presented evidence could make things interesting for those on the paleoecology side of this issue.
Outreach should be embraced. Get the science to the people!- Science funding is at a dangerous crossroads in the US. Either we show the public why our research is relevant and drum up support or we watch our grants dry up and our science with it. Our research is publicly funded and we have an obligation to share it with the taxpayer and get them involved.
Finally, never admit to not knowing who Steve Squyres is during a game of Cards Against Humanity, the Mars edition. I learned this the hard way and I will forever be grateful to those who didn’t toss me out of the game in spite of my ignorance.
Post Winter term musings and LPSC 2013!
Currently I’m sitting in the Portland airport thinking about the whirlwind of a term that just ended. If I could sum up this term into a single phrase, it’d be the one that a few friends have said: I don’t know how you do it. And to be honest, I’m not sure either. This term the Freethinkers, the campus skeptic group, helped co-sponsor and organize a talk by noted Interfaith/queer/atheist speaker, Chris Stedman. As one of the co-officers I had to communicate with local CFI leaders and coordinate with the school to pull off such an event. There were other activities we were involved in as well- all of which I received substantial support from school advisers and other members of the group.
While that was going, I was working with my other adviser, Melinda Hutson, on building our poster for the Lunar and Planetary Science Conference in Houston. It was a lot of work, but it looks great and I’m excited to present our findings to whomever wonders their way over to my little section of the conference venue. Here’s where you can see the poster and it’s accompanying abstract.
As I was working on that, I applied for and received a scholarship through the McNair foundation that will allow me to conduct research this summer. There’s a generous stipend involved and I’ll be able to pretty much do full time research that will go towards the paper that will discuss my meteorite. Did I mention that while all this was going on I had my studies to attend to, as well? And I have a part time job.
Just when I thought no term could be busier than the Fall, Winter comes along and proves me wrong.
But you know what? I loved it (for the most part). I could have done with more sleep, but I got everything done. The last two weeks have been especially brutal as I had to finish all my finals and projects a week early in order to attend my first ever LPSC. And now, I sit in the airport waiting for my flight to Houston. There I will have the chance to network and learn from others in my field and gain a better perspective of what it takes to grow and succeed in the planetary sciences.
I’m going to be one of the microbloggers and I’ll be tweeting all the cool science that’s discussed at the various meetings. I plan on sticking with the meteorite related material, but there’s a whole session on science outreach for which I am particularly excited. You can find these tweets on this blogs homepage at the right side of the screen. I plan on doing some blog posts to sum up what I’m learning, as well.
All in all, I can’t image a better end to my term. Here’s hoping the Spring term will allow me to get back to a somewhat regular blogging routine.
Fireball spotted over Russia
Some pretty stunning footage is coming out of Russia of a possible fireball. I’m saying possible because some news outlets are also claiming that the Russians shot the thing down while in mid-flight. While I don’t doubt that a fireball was spotted over Russian skies, I do doubt the Russians ability to shoot it down. Such a task would require that the Russians knew of its entry well before it entered the earth’s atmosphere and produced an explosion. My feeling is that anything of that size would have been found by more agencies than just the Russian military. I’m gonna follow this pretty closely and see what comes of it all. Until then enjoy the video’s of the fireball and the accompanying explosion.
And the explosion…
Phil Plait, the Bad Astronomer, has put up an excellent preliminary article about the Russian fireball.
I’ll continue to add links and updates as they come through. Exciting stuff!
Geochem Brain Dump
I really shouldn’t be blogging right now. I should be doing my on-line coursework for Exploring Mars, but I’m mentally exhausted and just need to give my brain a break. So, I’m just gonna dump all my thermodynamics stuff here. I’ve spent the last couple hours staring at a white board in the meteorite lab trying to figure out this problem from geochemistry:
Calculate deltar G for reaction [1] at a temperature of 25 oC and a pressure of 5 Kb (~5000 atm) assuming pure carbonate phases, and that deltar V = constant with pressure. The molar volumes of aragonite and calcite are Voarag = 34.150 cm3/mol and Vocalcite = 36.934 cm3/mol. 1 cm3 = 0.0239 cal/bar. At T = 25 oC and P = 5 Kb, in which direction will reaction [1] /proceed, and which carbonate is more stable?
Reaction 1 refers to CaCO3 (aragonite) to CaCO3 (calcite)
We calculated the Gibbs free energy equation in order to determine which direction the reaction would proceed and which mineral would be stable at standard temperature and pressure. In this case, aragonite doesn’t do so hot at 1 bar of pressure and 298 K and actually converts to calcite in about 10,000,000 years.
The question I’d been working on asked us to look at the same reaction, but at 5,000 bars of pressure instead of 1 bar. For those unfamiliar with measurements of pressure, 1 bar is basically the amount of pressure we feel at sea level. So, we had to determine in which direction the reaction would occur and which mineral was stable at 5,000 bars of pressure. That simple change in pressure resulted in this:
It’s not easy to see here, but there are actually two different sets of calculations: the massive one that takes up the entire board and that small, simple boxed one in the upper right corner. For those that haven’t ran away screaming yet, the right answer is in the latter equation. At 5,000 bars of pressure calcite becomes aragonite because it’s more stable at higher pressures. And then I realized something- the answer was in the question. Aragonite takes up less volume per mole than calcite. Aragonite has the same structure as calcite, but takes up less space. And that’s because its only stable at pressures that essentially “squish” its structure. At one bar of pressure, aragonite essentially relaxes- very slowly- and takes up a little more room and becomes calcite.
Once I saw this I had a an “a-ha” and “duh” moment all at the same time. Had I remembered that little fact about aragonite taking up less space I would have had some idea what the correct answer was. Granted, I still needed to do the work, but I would have realized that I needed a positive Gibbs value in order for the reaction to occur from the left to the right. *long exasperated sigh*
/end brain dump.
Accretionary Wedge #54- On The Rocks: Geo-brews and Geo-cocktails
If you look up the word “geologist” on the internets, you’ll probably come across this entry from the Urban Dictionary. However, the most apt part of it is the following:
There is a considerable, and still growing body of scientific literature that suggests that geologists are in fact the world’s first alcohol-based life form.
Hence the theme of this month’s Accretionary Wedge #54- One The Rocks: Geo-brews and Geo-cocktails.
This idea came about as Michael Klass, Julian Lozos and I sat at a cocktail place in Portland discussing geology, beer and cocktails. Naturally, rock themed booze ideas came out of it and this AW was born.
Our submissions for this month were few, but mighty.
- Elli Goeke at Life In Plane Light shakes up a couple of great drinks in the form of a lava-rock filtered vodka from Iceland and a cocktail based on the Boston Molasses Flood.
- My fellow PNW blogger, Michael Klaas at the Cascadia Blog, decided on two submissions for this month’s theme: The Magma Chamber for those who like a little heat in their libations and a boozy coffee concoction worthy of any nightcap, or ahem… any start to one’s day.
- David Bressan at the History of Geology talks about the chemistry of the most important aspect of our preferred beverages: water.
And my submission for this booze soaked Wedge? Meteor Beer! This one comes to us from the Alsace region of North-East France. According to the website it’s one of the oldest breweries in the country and was founded in 1640. I went with this beer, not just because it’s named Meteor, but because the meteorite Orgueil was found in France. This meteorite is important because it belongs to class of meteorites whose chemical composition closely approximates that of the sun. We use it as a standard when looking at how the solar system evolved chemically over time. Meteor beer and Orgueil have something in common: they’re both precursors to that which would come afterwards in their respective realms.
I have a confession though: I haven’t tried this beer. Trying to hunt it down has been nearly impossible- even in the great beer capitol that is Portland, OR. We have an empty bottle of it down in the meteorite lab though and it’s my goal to try one at some point in my life.
If after reading these entries you feel inspired to write about your own geology themed drink, or you wrote one and I missed it, post it in the comments section below and I’ll add it to this Wedge.
Here’s a few extra posts to add to the Wedge:
A paleontology themed beer from Silver Fox over at Looking for Detachment.
Ann muses on her favorite beer, Rolling Rock.
Ian at Hypocentre gives us a look at how geology effects the chemistry of the water in the brewing powerhouses that are the British Isles and Czech Republic.
And @meaganhogg shares an ale that was brewed a little closer to home:
Ontherocks at the Geosciblog presents an impressive collection of geo themed beer cans.
Meteorite Monday: So you think you have a meteorite, part 2.
Part of my responsibilities at the meteorite lab is to handle the e-mails we receive concerning meteorite inquiries. These come in on a nearly daily basis and I can easily receive dozens of requests in a week. I don’t mind answering the e-mails as I consider it an integral part of the outreach that we do as a lab. And if I can teach people about what to look for in a meteorite (or meteorwrong in most cases), then all the better. Generally speaking people are pretty good at following our guidelines for e-mailing us: namely, send us a small amount of high resolution photos. We prefer quality over quantity. As I ranted earlier on Twitter, I occasionally receive e-mails with 25+ photos and it’s a temptation to just delete them and move on.
The sad truth is that the vast majority of people that contact me don’t have meteorites. I would love nothing more than to say “yes you have a meteorite!” to everyone that contacts me. Unfortunately, reality says that earth rocks are far more common than space rocks, but with the added challenge that they all look nearly the same. Take for example this one:
Either it has a fusion crust or it’s a weathering patina. And those indentions are either reglamglypts or weathering features. This is one of the few e-mails I’ve received where I had to stop and examine the picture more closely. Generally speaking I get these sort of pictures:
This rock has a shape that is indicative of being in a fluvial setting. Meteorites are not round and, as a general rule, do not contain vesicles. More importantly, meteorites don’t survive long in a fluvial setting and erode away faster than your typical river sediment.
Some of the worst offenders are slag. This is the byproduct of metal production and is routinely confused for being an iron meteorite. Sometimes slag isn’t even magnetic which just completely rules it out as a meteorite.
My favorite meteorwrongs are scoria. I don’t even need to look at the picture for long to know that it’s not a meteorite.
Could I be wrong in my visual assessments? Of course. Identifying meteorites via photo alone is like trying to distinguish between chocolate chip cookies and raisin cookies at a distance. It all looks the same until you’ve bitten into it and was sorely disappointed by the presence of raisins instead of chocolate. Yes, I have trust issues from such experiences, but that’s besides the point. I may not know with certainty what type of rock you have, but I know just enough to determine if it’s from space or not. And if I happen to be wrong, then all the better!
*for part 1, click here*